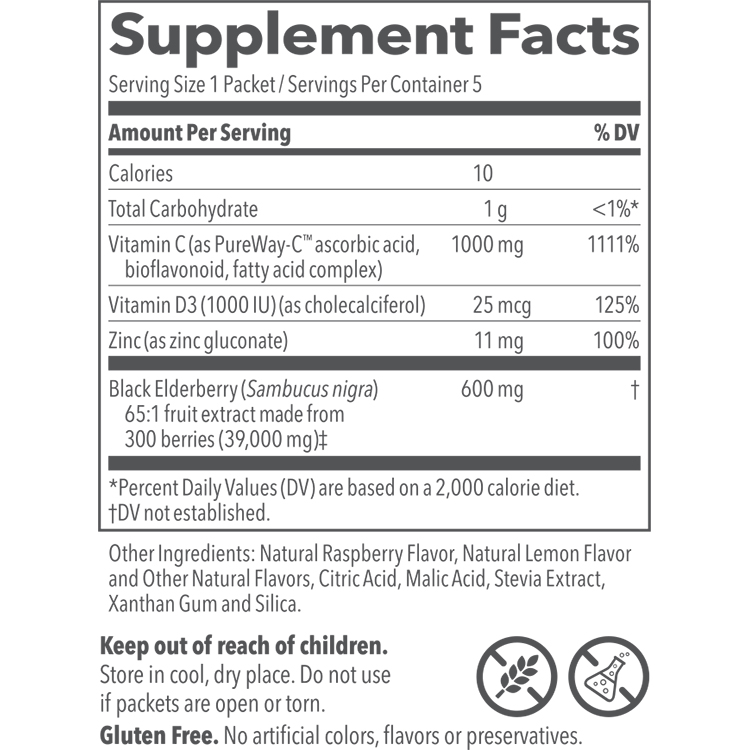
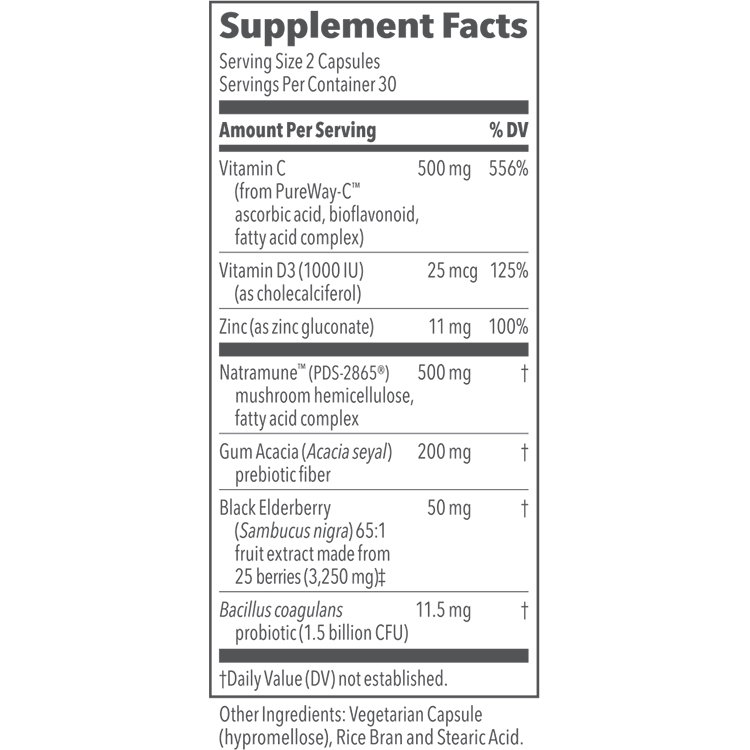
BACKGROUND
Ingredient Type: Botanical, Extract
Also Known As: Trigonella foenum-graecum, Methi, Bird’s foot, Fenogrego, Gray hay, Vendayam

Fenugreek is an aromatic, clover-like herb that is native to the Mediterranean region, southern Europe, and western Asia. The plant has foliage that grows to be 50 cm high and seed-containing pods that form as the plant matures (1). Fenugreek seeds have been used in cooking, as medicine, or to mask the taste of medicines (2). Some say that the taste and odor of fenugreek seed resemble maple syrup (3). Fenugreek is rich in many vitamins, minerals, and polysaccharides, and an amino acid that is believed to help lower the rate of glucose absorption in the intestines. Its leaves and seeds are commonly used as ingredients in traditional Indian foods (4).
The scientific species name for fenugreek, foenum-graecum, means “Greek hay.” The name was likely given to the herb because of its historical use as a forage crop (5).
TRADITIONAL USES
Ayurveda makes use of fenugreek’s dried leaves, known as Kasuri methi in Hindu. Kasuri methi is a staple spice in Indian culture and is used medicinally for gastrointestinal unease. Fenugreek seeds in powdered or extracted oil form are the primary source of medicinal fenugreek. It has been traditionally used for indigestion, hair loss, edema, enhance lactation, treat diabetes, and to control muscle spasms (6,7). Fenugreek was also used by ancient Egyptians as an ingredient in the mummification process. In modern Egypt, fenugreek is used to enrich baling flour (7,8). Ancient Romans used fenugreek as an aid in labor and delivery while ancient Chinese made use of it in treating general weakness (8).
WHAT DOES SCIENCE TELL US?
Fenugreek Possibly Decreases Appetite:
The literature is inconsistent with the effect of fenugreek on appetite. Studies of both strong and good scientific evidence have reported either decreases in appetite due to fenugreek consumption, or no significant effect.
A non-repeated single-blind, randomized, crossover study reported a decrease in appetite with hunger scores decreasing significantly at doses of 8g (p = 0.031) and 4g (p = 0.010) after 3.5 hours, n=18 (9). However, no changes were seen in leptin (a hormone involved in energy balance) levels in 45 male subjects who underwent an 8 week randomized, placebo-controlled trial ingesting 500mg of fenugreek daily (10).
The most scientifically robust study on the topic is a non-repeated double-blind randomized, placebo-controlled, three-period cross-over trial of two different doses (588 and 1176mg per day) in 12 male subjects over 14 days. The study reported a minor but significant decrease in appetite through the assessment of dietary fat consumption (P = 0.032) (11). A similar means of hunger assessment was carried out in a non-repeated, double-blind, randomized, placebo-controlled parallel trial evaluating the fat intake of 31 overweight male subjects over 6 weeks. The 1176mg daily dose of fenugreek yielded no significant effect on appetite, but a significant between-group decrease in fat intake was observed post-treatment with the treatment group (P = 0.032) (12).
Fenugreek Probably Helps Support Balanced Blood Sugar Levels:
Fenugreek research is dominated by studies on diabetes mellitus. Strong, consistent evidence is available on the efficacy of fenugreek in decreasing blood glucose levels, but there isn’t any agreement on the effect of fenugreek on insulin levels or insulin sensitivity.
The research on the effect of fenugreek on insulin levels is inconsistent. The strongest evidence is presented by a triple-blind randomized placebo-controlled trial which yielded a significant decrease in serum insulin (p=0.03) in type 2 diabetes mellitus ingesting 10g/day fenugreek over an 8-week period (13). In a double-blind, randomized, placebo-controlled parallel trial, 1176mg of fenugreek were ingested by 39 overweight males for 6 weeks. The fasting serum insulin/blood glucose ratio declined significantly in the treatment group (0.89 ± 0.09 (n = 19) vs 1.06 ± 0.10 (n = 19) mUI.mmol-1, P = 0.044), possibly due to concurrent increases in insulin sensitivity (11).
However, a significant insulin increase was observed in a non-repeated single-blind, randomized, crossover study in which peak insulin was greater in 18 obese subjects after consuming 8g of insulin as compared to 0g (P=0.01) (9). In another study, fenugreek yielded no significant changes in insulin after 45 resistance-trained men ingested 500mg for 8 weeks (10).
The literature on insulin sensitivity is similarly inconsistent with two studies of strong evidence showing either increases or no effect with the consumption of 1g/day fenugreek. One study was carried out on 25 type 2 diabetes mellitus (T2DM) patients and presented a minor but significant increase in insulin sensitivity (p<0.05) (14). The second study was carried out on 58 women with polcystic ovarian syndrome (PCOS) for 8 weeks, yielding no significant change in insulin sensitivity (15).
More agreement is seen on the effect of fenugreek on glycaemic control. 10/11 studies reported a significant decrease in blood glucose with the use of fenugreek; the 11th article reported no significant effect in 8 diabetics, 4 hours after ingesting 2.5g fenugreek. Strong, good and weak evidence such as a multicenter, randomized, placebo-controlled, double-blind, add-on clinical study; a randomized controlled cross-over study; and a cohort study, all present significant decreases in blood glucose with fenugreek dosages ranging from 0.5g to 100g (14,15,16,17,18,19,20,21,22,23,24,25). The most scientifically robust study saw a significant reduction in both fasting (21.98%) and postprandial (30.4%) blood sugar levels after 90 days with a 500mg dose, n= 108M 46F, (p<.001) (20).
Fenugreek Possibly Decreases Fat Mass and Triglycerides and Supports Good Cholesterol Levels:
There is mostly consensus in the literature that fenugreek has no significant effect on levels of HDL-C. Strong evidence (three randomized, double-blind, placebo-controlled trials) is presented with sample sizes as ranging from 25-120 and dosages ranging from 600mg-100g for healthy subjects as well as patients with type 1 and type 2 DM (19,26,27). A fourth study of less scientific robustness in which 20 hypocholesterolemic subjects consumed either 12.5g or 18.0g of germinated fenugreek seed powder over 30 days also saw no significant change in HDL (28). However, a randomized, placebo-controlled study of 25 type 2 DM patients ingesting 1g/day of hydroalcoholic fenugreek extract experienced a significant increase in HDL as compared to the control group (p<.05) (14).
There is insufficient evidence for the action of fenugreek on fat and lean mass. One double-blind, placebo-controlled trial found that parallel groups of resistance-trained men (n=30) experienced a decrease in fat mass of 1.77% compared with 0.55% in the non-treatment group (p<0.05) as well as significant effects for a time which were observed in regards to lean body mass (29).
There is inconsistent evidence on the effect of fenugreek on triglyceride levels with 3/6 studies purporting no significant effect and the other 3 reporting significant decreases. 600mg-18g were used in studies with from 20-120 subjects, for 30 days to 12 weeks, all reporting no significant changes to triglyceride levels (26,27,28). However, cohort studies and double-blind, placebo-controlled clinical trials both show significant decreases in triglycerides with the use of 1g-60g doses of fenugreek over 6-12 weeks. The most scientifically robust study resulted in a -9.2% (P < 0.001) incremental difference in triglyceride levels between the treatment and control group after 12 weeks ingesting 60g/day fenugreek (n=61) (24).
Fenugreek Might Decrease Pain:
The literature shows fenugreek to be effective in decreasing the symptoms of dysmenorrhea. A double-blind, randomized, placebo-controlled study reported decreases in pain duration, systematic menstruation symptoms and number of painkilling pills ingested (p<0.001) for an 1800-2700mg thrice daily dose of fenugreek after 60 days (n=101) (30). An open-label, randomized, standard-controlled study lasting 3 days reported a 66.89% reduction in abdominal pain from a 6g/day dose of fenugreek (31).
Fenugreek Might Help Support Aging Women:
Despite fenugreek’s historical use in feminine medicine, the literature does not hold a consensus on the effect of fenugreek on estrogen. It does, however, support the use of fenugreek to relieve menopausal symptoms and to increase libido.
Two double-blind, randomized, placebo-controlled studies show fenugreek to be effective in reducing the symptoms of menopause. One study used a dose of 600mg/day for 12 weeks on 115 health females resulting in a significant decrease in menopausal symptoms in the treatment group (12.4 ± 4.0) vs. (17.1 ± 5.5) in the placebo group (p < 0.001) (27). The second study reported significant decreases in hot flashes by 47.8% (p<0.001) and irritability and vaginal dryness by 50% (p<0.001) among other symptoms. The study made use of 1g/day over 90 days (n=88) (32).
Estrogen levels have seen inconsistent increases in fenugreek studies. Two randomized, double-blinded, placebo-controlled studies have presented significant increases in estradiol after ingesting fenugreek. One of the supporting studies utilized 600mg/day over 8 weeks in 80 low-libido healthy-weight menstruating women (p=.013) (33). The second study made use of a 1g/day dose in 88 healthy menopausal women over 90 days resulting in a 120% increase in estradiol (p<.01) (32). The non-supporting studies were similarly scientifically robust trials using 500mg-600mg fenugreek over 8-12 weeks with populations ranging from 30M-115F (10,27,29).
Fenugreek Might Help Increase Libido:
The literature agrees that fenugreek use is associated with increases in libido. Three double-blind, placebo-controlled trials all using 600mg/day doses over 6,8, and 12 weeks all showed significant increases in libido. The 6-week study with 60 men of varying BMI resulted in significant increases in orgasm, sexual cognition/fantasy, sexual behavior/experiences and sexual arousal (p<0.01) (34). In the 8-week study, 80 menstruating low-libido women of healthy weight experienced significant increases in sexual arousal (p=0.026) (33). In the 12-week study, 120 healthy men experienced a significant increase in total sexual drive (p=0.007) (26).
Fenugreek Possibly Increases Production of Breast Milk:
The literature on the use of fenugreek to enhance breast milk production is inconsistent, despite its historical association with breast milk increase. Two double-blind placebo-controlled studies resulted in no significant changes in breast milk production with dosages of 600mg and 1725g fenugreek/day over 6 weeks and 21 days respectively (34,35). However, three equally scientifically-robust studies all showed significant increases in breast milk production with the use of fenugreek. Two of these studies made use of fenugreek tea. Breast milk was roughly doubled (p=.004) in one study in which 66 new mothers were given 600ml humana fenugreek tea daily for 7 days or less (36). In the other study, baby weight showed a significant increase for babies of mothers consuming 22.5g/day fenugreek tea over a 4-week period (37). The third supporting study saw an increase in breast milk production in the treatment group compared to the control group (p = 0.0046) after day 5 in 36 postpartum women consuming either a placebo or 1.83g fenugreek capsules daily (38). It is noteworthy that drinking is significantly different from consuming standardized dosages of fenugreek such as in capsules. Such differences should be taken into consideration when prescribing dosages and ingestion methods.
Fenugreek Might Increase Testosterone:
The literature is inconsistent, but to some extent (5/7 studies) supportive of the use of fenugreek to increase testosterone levels. A randomized, placebo-controlled trial and a non-repeated, placebo-controlled, double-blind trial both showed no significant effect of fenugreek on testosterone, although the former trial did report a 9.42% decrease in dihydrotestosterone (DHT) for the treatment group vs. a 5.98% increase in the control group (p<0.05). This trial used 500mg/day over 8 weeks in 45 resistance-trained males (10). The latter study made use of 600mg/day in 60 males of varied BMI over a 6-week period (34).
In support of fenugreek’s effect on testosterone levels are studies of good scientific evidence such as 3 randomized, placebo-controlled, double-blind studies (26,33,39). The five total studies supporting fenugreek’s enhancing effect on testosterone levels all used either 500mg/day or 600mg/day doses, with durations from 20 hours to 12 weeks. Populations ranged from 16M to 120M. An 8-week study on 30 resistance-trained males showed a significant increase in total testosterone (p=0.018) and bioavailable testosterone levels (p=.049) (29). An 8-week study of 80 low-libido healthy-weight menstruating females reported a significant increase in free testosterone (p=0.043) (33). A 12-week study of 120 healthy males presented significant enhancement of total testosterone (p<0.001), and free testosterone (p<.002) (26). 50 males in a 12-week study reported a 147% increase in free testosterone levels (p<.001) and significant increases in sperm morphology and sperm count, but no significant change in total testosterone (40). A significant increase was presented in total, bioavailable, and calculated free testosterone but not in serum free testosterone by a 20-hour study in 16 males using 600mg/day fenugreek (39).
SAFETY
Fenugreek is considered to be safe for human ingestion (41).
Interactions:
Major
- A 32-year-old woman developed toxic epidermal necrolysis (TEN) after self-diagnosing poor lactation with fenugreek and co-ingesting painkillers. After two weeks, the patient was restored through a high dose of human immunoglobulins. Her allergological report suggests that fenugreek was the cause of her TEN (42).
- Another female patient appeared to suffer an adverse medical effect from fenugreek when the anticoagulant effects of her warfarin medication for cardiac arrhythmia were seemingly enhanced by the consumption of a combination of boldo and fenugreek (43). This indicates that the use of fenugreek should be monitored in patients with pre-existing medical conditions and medical treatments.
- Persons with peanut allergies may present a cross-reactive allergy to fenugreek (44).
Side-Effects:
- Fenugreek has been associated with pseudo maple syrup urine disease in which subjects’ urine emits a sickly-sweet scent due to the presence of sotolone, which is found naturally in fenugreek (38,45).
- Fenugreek has been associated with mild cases of gastrointestinal discomfort (29,33,34,46,47), dizziness (17,26), increased urination (17), headaches (26,33,38), and breast tenderness (38).
- It bears well to note that fenugreek has been implicated in clinical changes in levels of blood glucose, blood serum triglycerides, insulin sensitivity, and blood serum hormones (9,17,24,29,40).
- Fenugreek may lead to decreases in blood potassium levels (17).
REFERENCES
- Reeder C, Legrand A, O’Connor-Von SK. The Effect of Fenugreek on Milk Production and Prolactin Levels in Mothers of Preterm Infants. Clinical Lactation 2013;4(4):159-165
- Neelakantan N, Narayanan M, de Souza RJ, van Dam RM. Effect of fenugreek (Trigonella foenum-graecum L.) intake on glycemia: a meta-analysis of clinical trials. Nutr J 2014;13:7
- Bartley GB, Hilty MD, Andreson BD, et al. “Maple-syrup” urine odor due to fenugreek ingestion. N Engl J Med 1981;305:467.
- Patil SP, Niphadkar PV, Bapat MM. Allergy to fenugreek (Trigonella foenum graecum). Ann Allergy Asthma Immunol 1997;78:297-300.
- Yoshinari, O. and Igarashi, K. Anti-diabetic effect of trigonelline and nicotinic acid, on KK-A(y) mice. Curr Med Chem 2010;17(20):2196-2202.
- Singh J. Fenugreek Seeds (Methi) – Trigonella Foenum-Graecum Benefits, Uses & Side Effects. Ayur Times. https://www.ayurtimes.com/fenugreek-methi-trigonella-foenum-graecum/. Accessed February 26, 2018.
- Nagulapalli Venkata KC, Swaroop A, Bagchi D, Bishayee A. A small plant with big benefits: Fenugreek ( Trigonella foenum-graecum Linn.) for disease prevention and health promotion. Mol Nutr Food Res. 2017;61(6):1600950. doi:10.1002/mnfr.201600950
- Basch E, Ulbricht C, Kuo G, Szapary P, Smith M. Therapeutic Applications of Fenugreek. Altern Med Rev ◆ J Herb Pharmacother Chief Ed Massachusetts Gen Hosp Prim Outpatient Med. 2003;8(1).
- Mathern JR, Raatz SK, Thomas W, Slavin JL. Effect of Fenugreek Fiber on Satiety, Blood Glucose and Insulin Response and Energy Intake in Obese Subjects. Phyther Res. 2009;23(11):1543-1548. doi:10.1002/ptr.2795
- Bushey B, Taylor L, Wilborn C, et al. Fenugreek Extract Supplementation Has No effect on the Hormonal Profile of Resistance-Trained Males. Int J Exerc Sci Conf Proc. 2009;2(1). https://digitalcommons.wku.edu/ijesab/vol2/iss1/13. Accessed February 18, 2018.
- Chevassus H, Molinier N, Costa F, Galtier F, Renard E, Petit P. A fenugreek seed extract selectively reduces spontaneous fat consumption in healthy volunteers. Eur J Clin Pharmacol. 2009;65(12):1175-1178. doi:10.1007/s00228-009-0733-5
- Chevassus H, Gaillard J-B, Farret A, et al. A fenugreek seed extract selectively reduces spontaneous fat intake in overweight subjects. Eur J Clin Pharmacol. 2010;66(5):449-455. doi:10.1007/s00228-009-0770-0
- Rafraf M, Malekiyan M, Asghari-Jafarabadi M, Aliasgarzadeh A. Effect of Fenugreek Seeds on Serum Metabolic Factors and Adiponectin Levels in Type 2 Diabetic Patients. Int J Vitam Nutr Res. 2015;84(3-4):0196-0205. doi:10.1024/0300-9831/a000206
- Gupta A, Gupta R, Lal B. Effect of Trigonella foenum-graecum (fenugreek) seeds on glycaemic control and insulin resistance in type 2 diabetes mellitus: a double-blind placebo controlled study. J Assoc Physicians India. 2001;49:1057-1061.
- Hassanzadeh Bashtian M, Emami SA, Mousavifar N, Esmaily HA, Mahmoudi M, Mohammad Poor AH. Evaluation of Fenugreek (Trigonella foenum-graceum L.), Effects Seeds Extract on Insulin Resistance in Women with Polycystic Ovarian Syndrome. Iran J Pharm Res IJPR. 2013;12(2):475-481.
- Kochhar A, Nagi M. Effect of Supplementation of Traditional Medicinal Plants on Blood Glucose in Non–Insulin-Dependent Diabetics: A Pilot Study. J Med Food. 2005;8(4):545-549. doi:10.1089/jmf.2005.8.545
- Abdel-Barry JA, Abdel-Hassan IA, Jawad AM, al-Hakiem MH. Hypoglycaemic effect of aqueous extract of the leaves of Trigonella foenum-graecum in healthy volunteers. East Mediterr Health J. 2000;6(1):83-88.
- Losso JN, Holliday DL, Finley JW, et al. Fenugreek Bread: A Treatment for Diabetes Mellitus. J Med Food. 2009;12(5):1046-1049. doi:10.1089/jmf.2008.0199
- Sharma RD, Raghuram TC, Rao NS. Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur J Clin Nutr. 1990;44(4):301-306.
- Verma N, Usman K, Patel N, et al. A multicenter clinical study to determine the efficacy of a novel fenugreek seed (Trigonella foenum-graecum) extract (FenfuroTM) in patients with type 2 diabetes. Food Nutr Res. 2016;60:32382. doi:10.3402/FNR.V60.32382
- Kassaian N, Azadbakht L, Forghani B, Amini M. Effect of Fenugreek Seeds on Blood Glucose and Lipid Profiles in Type 2 Diabetic Patients. Int J Vitam Nutr Res. 2009;79(1):34-39. doi:10.1024/0300-9831.79.1.34
- Robert SD, Ismail AA, Rosli WIW. Reduction of postprandial blood glucose in healthy subjects by buns and flatbreads incorporated with fenugreek seed powder. Eur J Nutr. 2016;55(7):2275-2280.doi:10.1007/s00394-015-1037-4.
- Suchitra MR, Parthasarathy S. Effect of administration of fenugreek seeds on HbA1C levels in uncontrolled diabetes mellitus – a randomized controlled trial. Int J PharmTech Res. 2015;8(2):180-182.
- Fedacko J, Singh RB, Niaz MA, Ghosh S, Fedackova P, Tripathi AD, Etharat A, Onsaard E, Singh VK SS. Fenugreek seeds decrease blood cholesterol and blood glucose as adjunct to …: EBSCOhost. World Heart Journal. http://web.b.ebscohost.com.periodicals.sgu.edu/ehost/detail/detail?vid=13&sid=01513929-dc22-4298-ba7c-46718bbff267%40sessionmgr103&bdata=JnNpdGU9ZWhvc3QtbGl2ZQ%3D%3D#AN=CN-01328092&db=cgh. Published 2016. Accessed February 25, 2018.
- Lu F, Shen L, Qin Y, Gao L, Li H, Dai Y. Clinical Observation on Trigonella Foenum-graecum L. total saponins in combination with sulfonylureas in the treatment of type 2 diabetes mellitus. Chin J Integr Med. 2008;14(1):56-60. doi:10.1007/s11655-007-9005
- Rao A, Steels E, Inder WJ, Abraham S, Vitetta L. Testofen, a specialised Trigonella foenum-graecumseed extract reduces age-related symptoms of androgen decrease, increases testosterone levels and improves sexual function in healthy aging males in a double-blind randomised clinical study. Aging Male. 2016;19(2):134-142. doi:10.3109/13685538.2015.1135323
- Steels E, Steele ML, Harold M, Coulson S. Efficacy of a Proprietary Trigonella foenum-graecum L. De-Husked Seed Extract in Reducing Menopausal Symptoms in Otherwise Healthy Women: A Double-Blind, Randomized, Placebo-Controlled Study. Phyther Res. 2017;31(9):1316-1322. doi:10.1002/ptr.5856.
- Sowmya P, Rajyalakshmi P. Hypocholesterolemic effect of germinated fenugreek seeds in human subjects. Plant Foods Hum Nutr. 1999;53(4):359-365.
- Wilborn C, Taylor L, Poole C, Foster C, Willoughby D, Kreider R. Effects of a purported aromatase and 5α-reductase inhibitor on hormone profiles in college-age men. Int J Sport Nutr Exerc Metab. 2010;20(6):457-465.
- Younesy S, Amiraliakbari S, Esmaeili S, Alavimajd H, Nouraei S. Effects of Fenugreek Seed on the Severity and Systemic Symptoms of Dysmenorrhea. J Reprod Infertil. 2014;1515(11):41-4841.
- Inanmdar W, Sultana A, Mubeen U, Rahman K. Clinical efficacy of Trigonella foenum graecum (Fenugreek) and dry cupping therapy on intensity of pain in patients with primary dysmenorrhea. Chin J Integr Med. May 2016. doi:10.1007/s11655-016-2259-x
- Begum SS, Jayalakshmi HK, Vidyavathi HG, et al. A Novel Extract of Fenugreek Husk (FenuSMART TM ) Alleviates Postmenopausal Symptoms and Helps to Establish the Hormonal Balance: A Randomized, Double-Blind, Placebo-Controlled Study. doi:10.1002/ptr.5680
- Rao A, Steels E, Beccaria G, Inder WJ, Vitetta L. Influence of a Specialized Trigonella foenum-graecum Seed Extract (Libifem), on Testosterone, Estradiol and Sexual Function in Healthy Menstruating Women, a Randomised Placebo-Controlled Study. Phyther Res. 2015;29(8):1123-1130. doi:10.1002/ptr.5355
- Steels E, Rao A, Vitetta L. Physiological Aspects of Male Libido Enhanced by Standardized Trigonella foenum-graecum Extract and Mineral Formulation. Phyther Res. 2011;25(9):n/a-n/a. doi:10.1002/ptr.3360
- Reeder C, Legrand A, O’Connor-Von SK. The Effect of Fenugreek on Milk Production and Prolactin Levels in Mothers of Preterm Infants. Clin Lact. 2013;4(4):159-165. doi:10.1891/2158-0782.4.4.159
- Turkyılmaz C, Onal E, Hirfanoglu IM, et al. The Effect of Galactagogue Herbal Tea on Breast Milk Production and Short-Term Catch-Up of Birth Weight in the First Week of Life. J Altern Complement Med. 2011;17(2):139-142. doi:10.1089/acm.2010.0090
- Ghasemi V, Kheirkhah M, Vahedi M. The Effect of Herbal Tea Containing Fenugreek Seed on the Signs of Breast Milk Sufficiency in Iranian Girl Infants. Iran Red Crescent Med J. 2015;17(8):e21848. doi:10.5812/ircmj.21848
- Brillante C, Mantaring J. O-203 Triple-blind, Randomised Controlled Trial On The Use Of Fenugreek (trigonella Foenum-graecum L.) For Augmentation Of Breastmilk Volume Among Postpartum Mothers. Arch Dis Child. 2014;99(Suppl 2):A101.2-A101. doi:10.1136/archdischild-2014-307384.271
- Mokashi M, Singh-Mokashi R, Mohan V, Thakurdesai P. Effects of Glycosides Based Fenugreek Seed Extract on Serum Testosterone Levels of Healthy Sedentary Male Subjects: An Exploratory Double-Blind, Placebo-Controlled, Crossover Study. Vol 7. Innovare Academics; 2014. https://innovareacademics.in/journals/index.php/ajpcr/article/view/1108. Accessed February 25, 2018.
- Maheshwari A, Verma N, Swaroop A, et al. Efficacy of Furosap TM, a novel Trigonella foenum-graecum seed extract, in Enhancing Testosterone Level and Improving Sperm Profile in Male Volunteers. Int J Med Sci. 2017;14(1):58-66. doi:10.7150/ijms.17256
- CFR – Code of Federal Regulations Title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.20. Accessed February 25, 2018.
- Bentele-Jaberg N, Guenova E, Mehra T, et al. The Phytotherapeutic Fenugreek as Trigger of Toxic Epidermal Necrolysis. Dermatology. 2015;231(2):99-102. doi:10.1159/000433423
- Lambert JP, Cormier J. Potential interaction between warfarin and boldo-fenugreek. Pharmacotherapy. 2001;21(4):509-512.
- Fæste CK, Namork E, Lindvik H. Allergenicity and antigenicity of fenugreek (Trigonella foenum-graecum) proteins in foods. J Allergy Clin Immunol. 2009;123(1):187-194. doi:10.1016/j.jaci.2008.09.012
- Korman SH, Cohen E, Preminger A. Pseudo-maple syrup urine disease due to maternal prenatal ingestion of fenugreek. J Paediatr Child Health. 2001;37(4):403-404.
- Chevassus H, Gaillard J-B, Farret A, et al. A fenugreek seed extract selectively reduces spontaneous fat intake in overweight subjects. Eur J Clin Pharmacol. 2010;66(5):449-455. doi:10.1007/s00228-009-0770-0
- Gupta A, Gupta R, Lal B. Effect of Trigonella foenum-graecum (fenugreek) seeds on glycaemic control and insulin resistance in type 2 diabetes mellitus: a double-blind placebo controlled study. J Assoc Physicians India. 2001;49:1057-1061.
See the National Center for Complementary and Integrative Health entry for fenugreek, the Examine.com entry for fenugreek, the Michigan Medicine Health Library entry for fenugreek, this European Medicines Agency monograph on Trigonella foenum-graecum, or the RXList entry for fenugreek for more information.